Pressure and
temperature of the atmosphere obtained from the gases that make up it.
Author; Rogelio Pérez C
Summary;
There is a global problem known as climate change,
which consists of an increase in the temperature of the atmosphere, the main
focus on this problem is a theory known as the greenhouse effect, This
greenhouse effect is attributed almost 33°C of average temperature on the
planet. The main greenhouse gases are CO2 and methane, which are also the ones
with the least volume in the atmosphere, which is contrary to all known science
for the temperature of a gas. As the problem is the increase in the temperature
of the atmosphere, and this is a gas, i will use the laws of the gases to
present the contributions of the temperature and pressure for each of the main
gases that make up the atmosphere in dry air.
Introduction
There is a global problem known as climate change,
which consists in the increase of temperature of the atmosphere, the science
understands this problem from the hypothesis of a greenhouse effect caused by
certain gases; This greenhouse effect is attributed almost 33oC of average
temperature on the planet. The main greenhouse gases being CO2 and methane, but
as the atmosphere is a gas, the explanation of its temperature can be obtained
with charles Law for gases, this relate
the temperature of a gas to its volume, the theory says; For any gas the ratio
of temperature to volume are directly proportional, but there are other laws of
gases, boyle's law relates the pressure of a gas to its volume, Lussac's gay
law governs the relationship between the pressure of a gas and its temperature,
and these combined laws put the three together temperature,, pressure and
volume of gases, but as the amount of gas must be constant, Avogadro's law
relates the volume and quantity of a gas, in moles. And when we combine the 4
laws we get the ideal law of gases, with this law I will explain the
temperature and pressure that contribute for each of the gases that make up the
atmosphere, in dry air.
Theory;
The Greenhouse Effect. If the earth only absorbed
radiation from the sun without giving an equal amount of heat back to space by
some means, the planet would continue to warm up until the oceans boiled. We
know the oceans are not boiling, and surface thermometers plus satellites have
shown that the earth's temperature remains roughly constant from year to year
(the interannual globally-averaged variability of about 0.2 C or the 0.5 C
warming trend in the 20th century, notwithstanding). This near constancy
requires that about as much radiant energy leaves the planet each year in some
form as is coming in. In other words, a near-equilibrium or energy balance has
been established. The components of this energy balance are crucial to the
climate.
All bodies with temperature give off radiant energy.
The earth gives off a total amount of radiant energy equivalent to that of a
black body -- a fictional structure that represents an ideal radiator -- with a
temperature of roughly -18 C (255 K). The mean global surface air temperature
is about 14 C (287 K), some 32 C warmer than the earth's black body
temperature. The difference is due to the well-established greenhouse effect. U.S. Standard Atmosphere. [1]
Charles' Law
Relationship between the temperature and volume of a
gas when the pressure is constant;
In 1787, Jack Charles first studied the relationship
between the volume and temperature of a gas sample at constant pressure and
observed that when the temperature was increased, the volume of the gas also
increased and that when the volume cooled it decreased. [2]
The mathematical expression of this law is:
VT=k
Boyle's Law
The relationship
between the pressure and volume of a gas when the temperature is constant was
discovered by Robert Boyle in 1662. Edme Mariotte also reached the same
conclusion as Boyle, but did not publish his works until 1676. This is why in
many books we find this law called the Law of Boyle and Mariotte.
Boyle's law states that
the pressure of a gas in a closed container is inversely proportional to the
volume of the container, when the temperature is constant. [3]
The mathematical
expression of this law is:
P⋅V=k
Gay-Lussac's law
The relationship
between the pressure and the temperature of a gas when the volume is constant
was stated by Joseph Louis Gay-Lussac in early 1800. Sets the relationship
between the temperature and pressure of a gas when the volume is constant,
Gay-Lussac found that at any time during this process, the pressure-temperature
ratio always had the same value: [4]
PT=kPT=k
Law of Avogadro
Relation between the amount of gas and its volume
This law, discovered by Avogadro in the early
nineteenth century, establishes the relationship between the amount of gas and
its volume when temperature and pressure are kept constant. Remember that the
amount of gas we measure in moles. [5]
We can also express the law of Avogadro like this:
Vn=k
The ideal gas law, also called the general gas equation, is the equation
of state of a ideal gas. It was first stated by Benoît Paul Émile Clapeyron in
1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's
law, and Gay-Lussac's law. The ideal gas law is often written in an empirical
form: [6]
PV=nRT
Where P, V and T are the pressure, volume and
temperature; n is the amount of substance; and R is the ideal gas constant. It
is the same for all gases. It can also be derived from the microscopic kinetic
theory, as was achieved (apparently independently) by August Krönig in 1856[7]
and Rudolf Clausius in 1857.[8]
The state of
an amount of gas is
determined by its pressure, volume, and temperature. The modern form of the
equation relates these simply in two main forms. The temperature used in the
equation of state is an absolute temperature: the appropriate SI unit is
the kelvin. .[9]
Common forms
The most frequently introduced forms are:
PV=nRT=nkBNAT
Where:
P is the pressure of the gas,
V is the volume of the gas,
n is the amount of substance of gas (also known as number of moles),
R is the ideal, or universal, gas constant, equal to the product of the
Boltzmann constant and the Avogadro constant,
KB is the Boltzmann constant
NA is the Avogadro constant
T is the absolute temperature of the gas.
In SI units, p is measured in pascals, V is measured in cubic metres, n is
measured in moles, and T in kelvins (the Kelvin scale is a shifted Celsius
scale, where 0.00 K = −273.15 °C, the lowest possible temperature). R has the
value 8.314 J/(K·mol) ≈ 2 cal/(K·mol), or 0.0821 l·atm/(mol·K).
Molar form
How much gas is present could be specified by giving the mass instead of
the chemical amount of gas. Therefore, an alternative form of the ideal gas law
may be useful. The chemical amount (n) (in moles) is equal to total mass of the
gas (m) (in kilograms) divided by the molar mass (M) (in kilograms per mole): [10]
n=m/M
By replacing n with m/M and subsequently introducing density ρ = m/V, we
get:
PV =m/M.RT
P=m/V.RT/M
P=p. R/M.T
Defining the specific gas constant Rspecific(r) as the ratio R/M,
P=p.R specific.T
This form of the ideal gas law is very useful because it links pressure,
density, and temperature in a unique formula independent of the quantity of the
considered gas. Alternatively, the law may be written in terms of the specific
volume v, the reciprocal of density, as Pv=R.specific.T.
Calculating molar mass, density,
pressure and temperature in the atmosphere in dry air;
|
GAS |
No. of Moles in 1 Mole Air |
Molecular Weight |
Mass/grams |
|
Nitrogen (N2) |
0,78084 |
28 |
21,86352 |
|
Oxygen (02) |
0,20951 |
32 |
6,70432 |
|
Argon (Ar) |
0,00934 |
40 |
0,3736 |
|
CO2 |
0,000415 |
44 |
0,01826 |
|
Neon (NE) |
0,00001818 |
20.17 |
0,000366691 |
|
Helium (HE) |
0,00000524 |
4 |
0,00002096 |
|
Methane(CH4) |
0,00000187 |
16.04 |
2,99948E-05 |
|
Total weight in 1 mole of air |
|
28,96011765 |
|
Density p=M/V
Mass with all gases=28960117645 Gram/mole/1000=0, 028960118 Kg/mole
V=23,6448
L/mole/1000=0,0236448 M³
p=0,
028960118 Kg/mole /0,0236448 M³
p=1,224798588kg/M³
Calculating atmospheric pressure
PV =m/M.RT
P=m/V.RT/M
P=p. R/M.T
p= Density = 1,224798588kg/M³
R. specific = 8.31432 J/(mole⋅K)
M= 0,028960118 Kg/mol
T=
Atmosphere temperature.288k o 15°C.
Then
P=p. R/M.T
P=1,224798588 kg/M³ * 8,314 J/(mole⋅K)/ 0,028960118 Kg/mole *288k
P=1,224798588
kg/M³ 287,0844691 J/(mole⋅K) Kg/mole 288k
P= pressure 101266,
7479 Jm³
Calculating atmosphere temperature
P=p.R/M.T
p.R/M.T=P
T=P/p.R/M
P=
pressure 101266, 7479Jm³
p=Density 1,224798588kg/M³
R. specific
= 8.31432 J/(mole⋅K)
Mass with all gases= 0,028960118Kg/mole
T=P/p.R/M
T=101266,
7479Jm³ /1,224798588 kg/M³ * 8,314 J/(mole⋅K) /0,028960118 Kg/mole
T=101266,
7479Jm³ /10,18297546 kg/M³/J/(mole⋅K)*0,028960118 Kg/mole
T=101266,
7479Jm³ / 351,6206523M³Jk
T= Atmosphere temperature.288k or 15°C
Calculating molar mass, density, pressure and
temperature in the atmosphere in dry air, without carbon dioxide and methane
gases;
|
GAS |
No. of Moles in 1 Mole Air |
Molecular Weight |
Mass/grams |
|
Nitrogen (N2) |
0,78084 |
28 |
21,86352 |
|
Oxygen (02) |
0,20951 |
32 |
6,70432 |
|
Argon (Ar) |
0,00934 |
40 |
0,3736 |
|
CO2 |
|
|
|
|
Neon (NE) |
0,00001818 |
20.17 |
0,000366691 |
|
Helium (HE) |
0,00000524 |
4 |
0,00002096 |
|
Methane(CH4) |
|
|
|
|
Total weight in 1 mole of air |
|
28,94182765 |
|
Density
p=M/V
Mass without GHG=28,94182765 Gram/mol=0,028941828 Kg/mol
V=23,6448 L/mol=0,0236448M³
p=0.028941828 Kg/mol /0,0236448M³
p=1,224025056 kg/M³
Calculating atmospheric pressure
PV =m/M.RT
P=m/V.RT/M
P=p. R/M.T
p= Density = 1,224025 kg/m³.
R. specific = 8.31432
J/(mole⋅K)
Mass with all gases=
0, 02896011 Kg/mol
T=
Atmosphere temperature.288k o 15°C.
Then;
P=p. R/M.T
P=1,224025056 kg/M³ * 8,314 J/(mole⋅K) /
0,02896011 Kg/mole *288k
P=1,224025056
kg/M³ *287,0844691 J/(mole⋅K) Kg/mole
*288k
P= pressure without GHG = 101202,792 Jm³
Pressure with all gases = 101266, 7479 Jm³
Difference= 63,92911032 Jm³
Calculating atmosphere temperature
P=p.R/M.T
p.R/M.T=P
T=P/p.R/M
P= pressure 101202,792 jm³
p=Density 1,224025056 kg/M³
R. specific = 8.31432
J/(mole⋅K). 8,314 J/(mole⋅K).
Mass without GHG= 0,028941828Kg/mole
T=P/p.R/M
T=101202,792
jm³ 1,224025056 kg/M³ 8,314 J/(mole⋅K). /0,028941828
Kg/mole
T=101202,792
jm³ / 10,17654432 kg/M³ J/(mole⋅K)./0,028941828 Kg/mole
T=101202,792
jm³ 351,6206523m³j/k
T= Atmosphere temperature without GHG=287,8181 k
Temperature with all gases=288k or 15°C
Difference= 0,181812729k.
Calculating molar mass, density, pressure and
temperature in the atmosphere in dry air, without Nitrogen (N2);
|
GAS |
No. of Moles in 1 Mole Air |
Molecular Weight |
Mass/grams |
|
Nitrogen (N2) |
|
|
|
|
Oxygen (02) |
0,20951 |
32 |
6,70432 |
|
Argon (Ar) |
0,00934 |
40 |
0,3736 |
|
CO2 |
0,000415 |
44 |
0,01826 |
|
Neon (NE) |
0,00001818 |
20.17 |
0,000366691 |
|
Helium (HE) |
0,00000524 |
4 |
0,00002096 |
|
Methane(CH4) |
0,00000187 |
16.04 |
2,99948E-05 |
|
Total weight in 1 mole of air |
|
7,096597645 |
|
Density
p=M/V
Mass without N2=7, 096597645 Gram/mole=0,007096598 Kg/mole
V=23,6448 L/mol=0,0236448M³
p=0.007096598kg/mole /0.0236448m³
p=0, 300133545 kg/M³
Calculating
atmospheric pressure
without N2
PV =m/M.RT
P=m/V.RT/M
P=p. R/M.T
p= Density = 0,
300133545 kg/M³
R. specific = 8.31432
J/(mole⋅K)
Mass with all gases= 0, 02896011 Kg/mol
T=
Atmosphere temperature.288k o 15°C.
Then;
P=p. R/M.T
P=0,300133545
kg/M³ * 8,314 J/(mole⋅K) / 0,02896011 Kg/mole *288k
P=0,300133545
kg/M³ *287,0844691 J/(mole⋅K) Kg/mole
*288k
P= pressure without N2=24815, 14623 Jm³
Pressure with all gases =101266, 7479
Jm³
Difference = 76451,60164 Jm³
Calculating atmosphere temperature without N2
P=p.R/M.T
p.R/M.T=P
T=P/p.R/M
P pressure= 24815, 14623 Jm³
p Density= 0, 300133545 kg/M³
R. specific = 8.31432 J/(mole⋅K)
Mass without N2 =0,007096598Kg/mole
T=P/p.R/M
T=24815,
14623 Jm³ /0, 300133545 kg/M³ *8,314 J/(mole⋅K). /0,007096598 Kg/mole
T=24815,14623
Jm³ /2,495310293 kg/M³ J/(mole⋅K)./
0,007096598 Kg/mole
T=24815,
14623 Jm³ / 351,6206523m³j/k
T= Atmosphere temperature without N2=70,57363k
Temperature with all gases=288k or 15°C
Difference = 217, 4263688 k
Calculating molar mass, density, pressure and
temperature in the atmosphere in dry air, without Oxygen (O2);
|
GAS |
No. of Moles in 1 Mole Air |
Molecular Weight |
Mass/grams |
|
Nitrogen (N2) |
0,78084 |
28 |
21,86352 |
|
Oxygen (02) |
|
|
|
|
Argon (Ar) |
0,00934 |
40 |
0,3736 |
|
CO2 |
0,000415 |
44 |
0,01826 |
|
Neon (NE) |
0,00001818 |
20.17 |
0,000366691 |
|
Helium (HE) |
0,00000524 |
4 |
0,00002096 |
|
Methane(CH4) |
0,00000187 |
16.04 |
2,99948E-05 |
|
Total weight in 1 mole of air |
|
22,25579765 |
|
Density
p=M/V
Mass without O2=22,25579765 Grams/mole =0, 022255798 Kg/mol
V=23,6448
L/mol=0,0236448M³
p=0,022255798
kg/mol / 0,0236448M³
p=0, 941255483 kg/M³
Calculating
atmospheric pressure
without O2
PV =m/M.RT
P=m/V.RT/M
P=p. R/M.T
p= Density = 0,
941255483 kg/M³
R. specific = 8.31432
J/(mole⋅K)
Mass with all gases= 0, 02896011Kg/mol
T=
Atmosphere temperature.288k o 15°C.
Then;
P=p. R/M.T
P= 0,
941255483 kg/M³ * 8,314 J/(mole⋅K) / 0,02896011 Kg/mole *288k
P=0,
941255483 kg/M³ *287,0844691 J/(mole⋅K) Kg/mole *288k
P= pressure without O2=77823,
33177Jm³
Pressure with all gases =101266, 7479
Jm³
Difference = 23443, 41609 Jm³
Calculating atmosphere temperature without O2
P=p.R/M.T
p.R/M.T=P
T=P/p.R/M
P pressure= 77823, 33177Jm³
p Density= 0, 941255483 kg/M³
R. specific = 8.31432 J/(mole⋅K)
Mass without O2 =0, 022255798 Kg/mole
T=P/p.R/M
T=77823,
33177 Jm³ / 0, 941255483 kg/M³ *8,314 J/(mole⋅K). /0,
022255798 Kg/mole
T=77823,
33177 Jm³ / 7,825598086 kg/M³ J/(mole⋅K)./ 0, 022255798 Kg/mole
T=77823,
33177 Jm³ / 351,6206523m³j/k
T= Atmosphere temperature without O2=221,3275337k
Temperature with all gases=288k or 15°C
Difference = 66,
6724663 k
Calculating molar mass, density, pressure and
temperature in the atmosphere in dry air, without Argon (Ar);
|
GAS |
No. of Moles in 1 Mole Air |
Molecular Weight |
Mass/grams |
|
Nitrogen (N2) |
0,78084 |
28 |
21,86352 |
|
Oxygen (02) |
0,20951 |
32 |
6,70432 |
|
Argon (Ar) |
|
|
|
|
CO2 |
0,000415 |
44 |
0,01826 |
|
Neon (NE) |
0,00001818 |
20.17 |
0,000366691 |
|
Helium (HE) |
0,00000524 |
4 |
0,00002096 |
|
Methane(CH4) |
0,00000187 |
16.04 |
2,99948E-05 |
|
Total weight in 1 mole of air |
|
28,58651765 |
|
Density
p=M/V
Mass without Ar=28,58651765 Grams/mole =0, 02858651 Kg/mol
V=23,6448
L/mol=0,0236448M³
p=0,
02858651 kg/mol / 0,0236448M³
p=1,208998073 kg/M³
Calculating atmospheric pressure without O2
PV =m/M.RT
P=m/V.RT/M
P=p. R/M.T
p= Density = 1,208998073 kg/M³
R. specific = 8.31432
J/(mole⋅K)
Mass with all gases= 0, 02858651 Kg/mol
T=
Atmosphere temperature.288k o 15°C.
Then;
P=p. R/M.T
P= 1,208998073
kg/M³ * 8,314 J/(mole⋅K) / 0,02896011 Kg/mole *288k
P=1,208998073
kg/M³ *287,0844691 J/(mole⋅K) Kg/mole
*288k
P= pressure
without Ar= 99960, 38257 Jm³
Pressure with all gases =101266, 7479 Jm³
Difference = 1306, 3653 Jm³
Calculating atmosphere temperature without Ar
P=p.R/M.T
p.R/M.T=P
T=P/p.R/M
P pressure= 99960, 38257 Jm³
p Density= 1,208998073 kg/M³
R. specific = 8.31432 J/(mole⋅K)
Mass without Ar =0, 02858651 Kg/mole
T=P/p.R/M
T=99960,
38257 Jm³ / 1,208998073 kg/M³ *8,314 J/(mole⋅K). /0,
02858651 Kg/mole
T=99960,
38257 Jm³ / 10,05160998 kg/M³ J/(mole⋅K)./ 0, 02858651 Kg/mole
T=99960,
38257 Jm³ / 351,6206523m³j/k
T= Atmosphere temperature without Ar=284,284731k
Temperature with all gases=288k or 15°C
Difference = 3,715268973 k
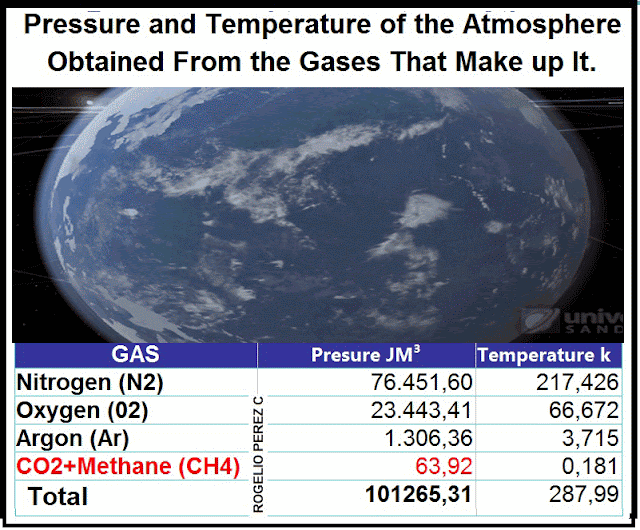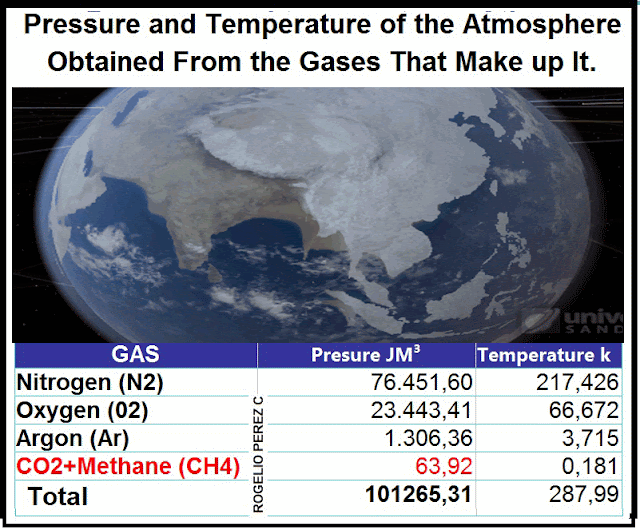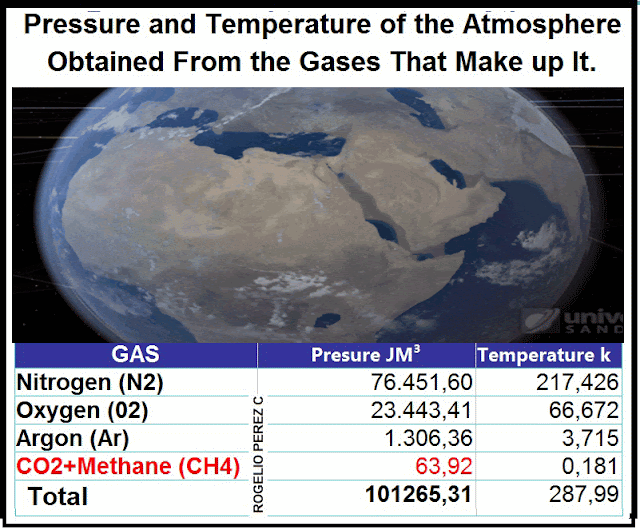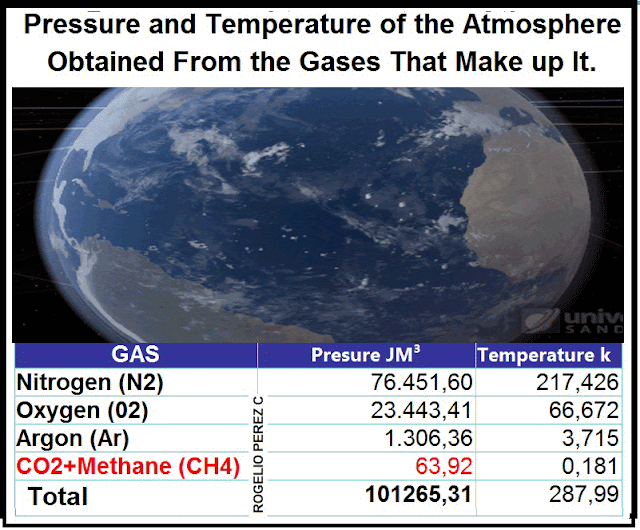
Contribution of each gas that
make up the atmosphere in Dry Air, to the pressure and temperature of the
planet
|
GAS |
Pressure
JM³ |
Temperature k |
|
Nitrogen
(N2) |
76451,60164 |
217,4263688 |
|
Oxygen
(02) |
23443,41609 |
66,6724663 |
|
Argon
(Ar) |
1306,3653 |
3,715268973 |
|
CO2+Methane
(CH4) |
63,92911032 |
0,181812729 |
|
Total in
pressure y temperature 1 mole of air |
101265,3121 |
287,995916832 |
Conclusion,
It can be concluded
that if we remove gases such as carbon dioxide and methane from the atmosphere,
the temperature of the atmosphere would be almost the same as what we currently
have.
It can also be
concluded that gases known as greenhouse gases do not contribute at 33°C to the
average temperature of the planet.
Finally we can say
that the strategy of removing carbon dioxide and methane from the planet's
atmosphere is not the best tool for combating heat climates.
Bibliography;
1 https://stephenschneider.stanford.edu/Mediarology/Statement_of_Stephen_Schneider_Climate_Change.html
2-http://www.educaplus.org/gases/ley_charles.html
3-https://www.educaplus.org/gases/ley_boyle.html
4-https://www.educaplus.org/gases/ley_gaylussac.html
5-http://www.educaplus.org/gases/ley_avogadro.html
6-https://en.wikipedia.org/wiki/Ideal_gas_law
7- Krönig, A. (1856). «Grundzüge einer Theorie
der Gase». Annalen der Physik 99 (10): 315-22. Bibcode:1856AnP...175..315K.
doi:10.1002/andp.18561751008. (en alemán) Facsimile at the Bibliothèque
nationale de France (pp. 315–22).
8- Clausius, R.
(1857). «Ueber die Art der Bewegung, welche wir Wärme nennen». Annalen der
Physik und Chemie. 3 176: 353-79. Bibcode:1857AnP...176..353C.
doi:10.1002/andp.18571760302.
9-Mendeleev, D. I. (1874). «О сжимаемости газов (En la
compresibilidad de los gases)». Russian
Journal of Chemical Society and the Physical Society 6: 309-352. (en ruso)
(From the Laboratory of the University of St. Petersburg).
10- Mendeleev, D. I. (1875). «Об упругости газов (En la compresibilidad de los gases)». Facsimile at the Bibliothèque nationale de France.
The greenhouse effect isn't that the glass get warm. It is that the air inside the glass get warm.
ResponderEliminarWe can experience a day / night temperature difference of up to 20°C under a clear sky. this shows for me, that there's no relevant blockage for outgoing radiation. How does this relate to the comparison to a greenhouse, which doesn't let its content cool out over night?
EliminarBECOME SMART AND GET RICH IN LESS THAN THREE DAYS...THIS IS NOT A JOKE
ResponderEliminarwithdraw $5000 dollars in one day with this card
i want everyone to be rich like me,i want everyone to partake on this new trick called programmed blank ATM card,i got a card from this hacking company that is why i am sharing the good news to the world,BLANK ATM CARDS ARE REAL, thank you. brainhackers@aol.com
website https://expertatmhackersrealmachineshackers.wordpress.com